The gasification process is conduct similar to pyrolysis (high process temperatures close to 1000 °C, as in the case of high-temperature pyrolysis), thus often both processes are confused with each other. Gasification consists of a series of complex sequential chemical reactions and thermal decomposition reactions.
The main difference between the pyrolysis and gasification processes is the addition of a gasifying agent to the gasified waste. It may be oxygen or air, but also water vapor, carbon dioxide or simply exhaust gas. Gasification takes place in the presence of O2, which results in the formation of dioxins and furans harmful to health. The dioxins and furans formed with the participation of oxygen very strongly disturb the hormonal balance of the body, leading to the occurrence of many dangerous diseases.
Some of the reactions occurring during the gasification process are exothermic (it does not require the supply of heat from the outside) and some of it is endothermic (requires the supply of heat from the outside). The gasification process is therefore more difficult to control, and its course depends on the gasifier used. Pyrolysis is an endothermic process. Gasification can also be seen as a continuation of the pyrolysis process, i.e. both processes may be successive stages, which increases the yield of energy gas. The gas generated in the gasification process is used directly for the production of electricity – burned in gas engines, or subjected to refining to the synthesis gas used in the synthesis of chemicals, including synthetic fuels. The calorific value of gas obtained as a result of gasification depends on the type of oxidizing agent and ranges from 5 MJ/m3 (for air and water vapor) to 10 MJ/m3 (for pure oxygen). For pyrolysis the calorific value of the obtained gas is from about 15 MJ/m3 even to over 30 MJ/m3 (depending on the substrate).
The main difference between the pyrolysis and gasification processes is the presence of an oxidizing agent in the process in the case of gasification and the calorific value of the gas obtained – higher calorific value in the case of pyrolysis.
Rys. Fig. Comparison of pyrolysis, gasification and incineration processes
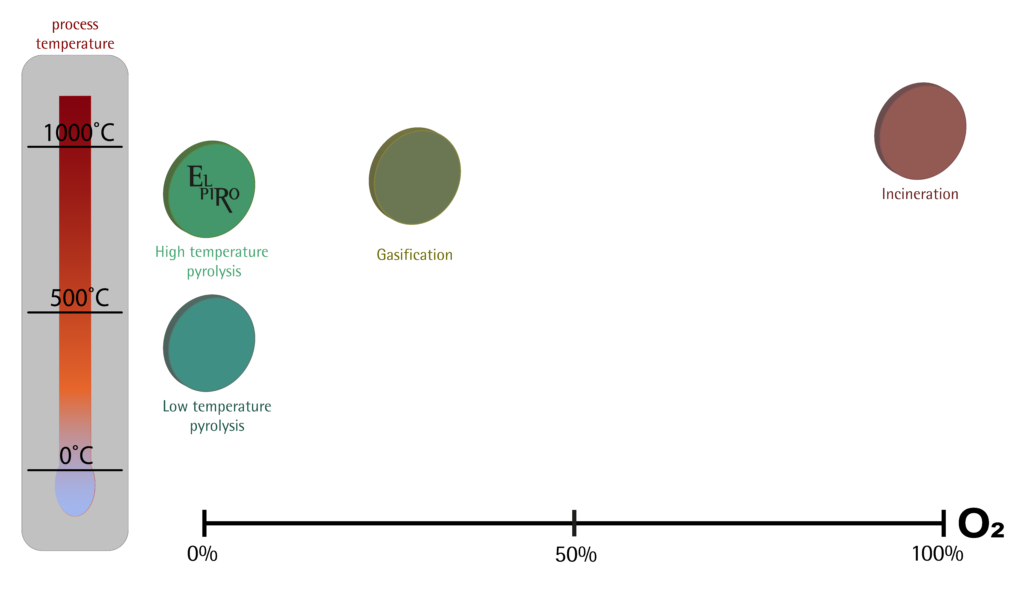
The resulting pyrolysis and gasification processes:
- High temperature pyrolysis (for example EL PIRO):
In this process, the main products are gas and biochar, heat is also generated
- Low temperature pyrolysis
In this process, the main products are oil, gas and biochar, heat is also generated
- Gasification
In this process, the main products are gas and biochar, heat is also generated, and due to the access of oxygen in the process – dioxins and furans
Źródła:
Introcuction to biorafineries and biofuels Assignment 8: comparison of gasification, pyrolysis and combustion, Aalto University School of Chemical Technology 2013
Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge to energy management in Greece, M.C. Samolada, A.A. Zabaniotou, Waste Management, 2014
Life cycle assessment of pyrolysis, gasification and inceneration waste-to-energy technologies: Theoretical analysis and case study of commercial plants, J. Dong, Y. Tang, A. Nzihou, Y. Chi, E. Weiss-Hortala, M. Ni, Science of the Total Environment, 2018
